CP (Control Plan) | Stable production requires the continuous control of process parameters and product characteristics. In order to reach defect-free production, the highest possible reduction of risks is necessary. Control means not only the detection of failures, but also the detection of variation in case of parameters over time.
Control Plan (CP) is a structured document, which sums up all process- and product characteristics. What are being monitored to ensure the capability of processes and the conformance of products to the relevant specifications. Additionally, built on the basis of a process flow chart, the CP contains the actions for each process step that are required to keep the process outputs in control.
CP is not a static, but a living document, and needs to be dynamically updated, as part of process improvement, based on DMAIC (Define, Measure, Analyze, Improve, and Control) principles.
Control Plan is an essential document in everyday manufacturing, and mandatory in the automotive sector. It is part of the Advanced Product Quality Planning (APQP) systematic framework. We differentiate pre-launch and serial CPs; however, the methods are the same. Pre-launch CP is the document, which is used in the APQP phase “Process design and development verification”, while it is updated to serial CP in the APQP phase “Product and process validation”.
FMEA & Control Plan
CP has very strong connection to FMEA, as it serves as the risk identification tool, which supports the elaboration of the CP by defining control actions, what are suitable to eliminate the process and product related risks. The following chart illustrates the place of Control Plan in the product engineering planning process:
The structure of Control Plan consists of two major sections: the header and the control matrix.
The header needs to include general information of the CP, for better identification and tracking, such as:
- Control Plan ID Number
- Customer.
- Site / Plant.
- Relevant Part Number (P/N) and Part Name or Description.
- Date of first release (original).
- Revision Level (e.g. A – first release, B – second release, etc.).
- Revision Table (containing all major updates with dates).
- Status (e.g. pre-launch CP, serial CP).
- Indication of special / critical characteristics (e.g. S – safety relevant characteristic).
- Name of responsible engineer / specialist (who prepared and updates the document).
- Core team (names of all team members, who sign off the release and the updates).
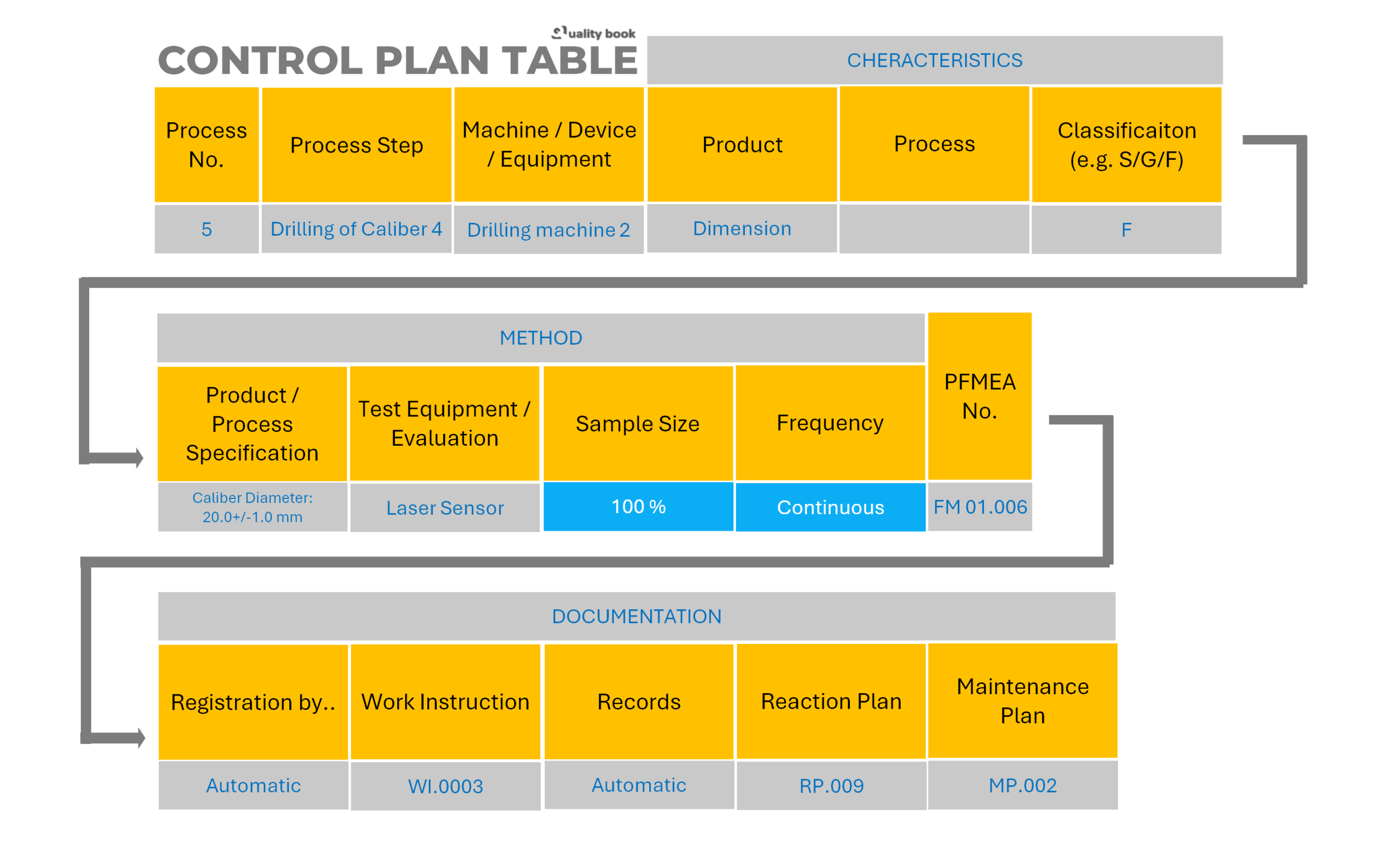
The matrix (content) of the Control Plan has to contain the following information in a table format:
- Column 1 – Process number.
- Column 2 – Process step (brief description of process, e.g. “Drilling of caliber 4”).
- Column 3 – Machine / device / equipment (the equipment, that performs the process step, e.g. “Drilling machine 2”).
- Column 4.1 – Characteristics – Product (e.g. “Dimension”).
- Column 4.2 – Characteristics – Process (e.g. “Soldering time-frame”).
- Column 4.3 – Characteristics – Classification (e.g. “S – Safety Relevant, G – Governmental Relevant, F – Function Relevant”).
- Column 5.1 – Methods – Product / Process specification (e.g. “Caliber diameter: 20,0 + / – 1,0 mm”).
- Column 5.2 – Methods – Test equipment / Evaluation (e.g. “Laser sensor”).
- Column 5.3 – Methods – Sample size (e.g. “100% or 2 pcs”).
- Column 5.4 – Methods – Frequency (e.g. “Continuous or Hourly”).
- Column 6 – FMEA number (the link between the CP and the PFMEA, e.g. “FM.01.006”).
- Column 7.1 – Documentation – Registration by (who registers the result of control, e.g. “Automatic, or Measurement technician”).
- Column 7.2 – Documentation – Work instruction (the relevant work instruction that describes the given process step, e.g. “WI.0003”).
- Column 7.3 – Documentation – Records (the physical or digital records that contain the measured process or product characteristic, e.g. “Machine log (automatic) or 3D.REC.001”).
- Column 7.4 – Documentation – Reaction Plan (the document, that describes what to do in case of discrepancy, e.g. “RP.009”).
- Column 7.5 – Documentation – Maintenance Plan (the document, that belongs to the given machine, equipment, e.g. “MP.0002 – Driller and Sensor”).
Product & Process
Control plan is a generally used document in many manufacturing business sectors (and also in service providing sectors), so it’s not a surprise it received various designations. CP is referred to under many names, but all cover the same substance:
- Serial Control Plan.
- Production Control Plan.
- Process Control Plan.
In order to create a proper CP, and maintain it as a living document, you should consider to take care of the following:
- PFMEA must be in a state ready for serial production, and all potential process related risks need to be understood by the team. Of course, FMEA is also a living document, thus it needs to be updated with further identified risks, however the team must do as much as possible to lift it to the highest state before Start of Production (SOP). Amend your CP in case of PFMEA change, or any change of process parameter or product characteristics.
- Product specifications and process parameters are precisely defined, and represent customer requirements.
- Use the “error proofing” principle that means you prevent defects at the origin. Prevention also means the decrease of scrap rate. In terms of control effectiveness, the following order applies: error proofing / failure preventions > 100% testing > sampling inspection.
- Many companies focus on control (sample size, frequency, etc.), but much less of them take care of effective reaction plans. Define optimum intervention limits, e.g. you react not only if you measure out of spec. product characteristics of process parameters, but you also react in case an anomaly or high variation is detected. Statistical Process Control (SPC) is in your aid.
- Use the Control Plan during process audits, and plan your frequent audits (yearly audit plan) to verify if your CP reflects the production circumstances and vice versa.
Conclusion
- CP stands for Control Plan.
- Control Plan is a structured document, which sums up all process- and product characteristics, what are being monitored to ensure the capability of processes and the conformance of products to the relevant specifications.
- In addition, CP contains the actions for each process step that are required to keep the process outputs in control.
- CP is an essential document of quality control, and part of the APQP framework.
- As part of quality improvement, CP must reflect process and product changes, and has to be updated as a living document (CP is never static).
- CP has a very close connection to PFMEA, as the controls are defined based on identified risks and failure modes.